Recent Publications (Original)
Unique amphipathic α helix drives membrane insertion and enzymatic activity of ATG3 (Nishimura et al., Sci Adv.)
2023.06.28 Recent Publications (Original)
Taki Nishimura*, Gianmarco Lazzeri, Noboru Mizushima, Roberto Covino*, Sharon A Tooze (*co-corresponding authors)
Unique amphipathic α helix drives membrane insertion and enzymatic activity of ATG3
Sci Adv. 2023 Jun 23;9(25):eadh1281. doi: 10.1126/sciadv.adh1281. Epub 2023 Jun 23.
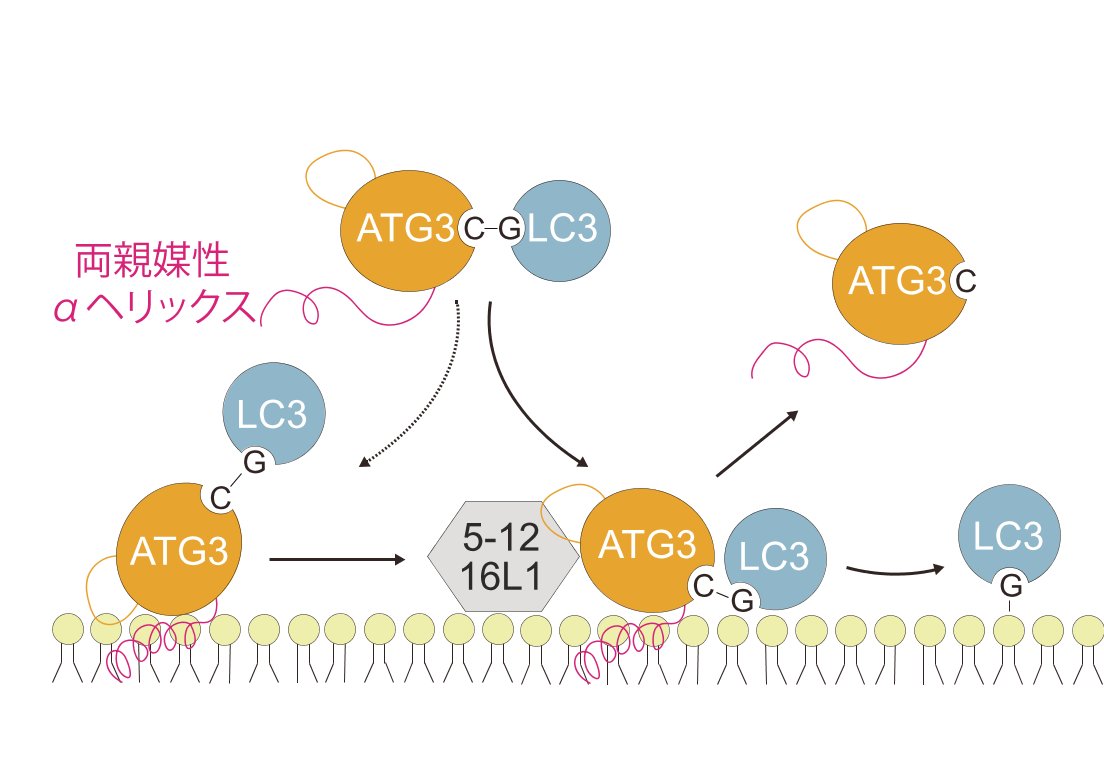
Autophagosome biogenesis requires a localized perturbation of lipid membrane dynamics and a unique protein-lipid conjugate. Autophagy-related (ATG) proteins catalyze this biogenesis on cellular membranes, but the underlying molecular mechanism remains unclear. Focusing on the final step of the protein-lipid conjugation reaction, the ATG8/LC3 lipidation, we show how the membrane association of the conjugation machinery is organized and fine-tuned at the atomistic level. Amphipathic α helices in ATG3 proteins (AHATG3) have low hydrophobicity and contain less bulky residues. Molecular dynamics simulations reveal that AHATG3 regulates the dynamics and accessibility of the thioester bond of the ATG3~LC3 conjugate to lipids, enabling the covalent lipidation of LC3. Live-cell imaging shows that the transient membrane association of ATG3 with autophagic membranes is governed by the less bulky-hydrophobic feature of AHATG3. The unique properties of AHATG3 facilitate protein-lipid bilayer association, leading to the remodeling of the lipid bilayer required for the formation of autophagosomes.