Recent Publications (Original)
Evolution and insights into the structure and function of the DedA superfamily containing TMEM41B and VMP1 (Okawa, Hama, Zhang et al., J Cell Sci)
2021.04.01 Recent Publications (Original)
Fumiya Okawa, Yutaro Hama, Sidi Zhang, Hideaki Morishita, Hayashi Yamamoto, Tim P. Levine, Noboru Mizushima
Evolution and insights into the structure and function of the DedA superfamily containing TMEM41B and VMP1
Jour Cell Sci, 26 March 2021 DOI: 10.1242/jcs.255877
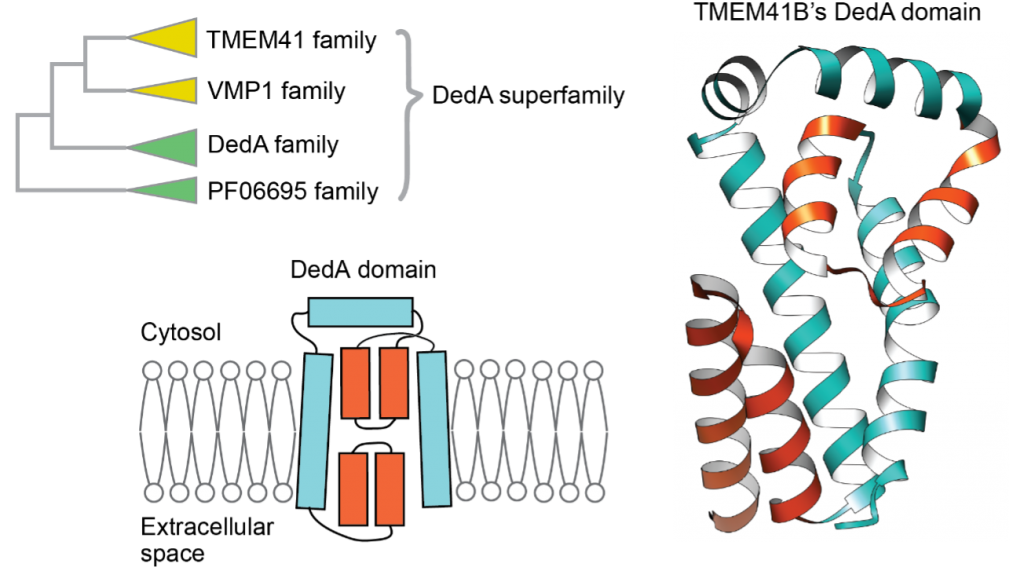
TMEM41B and VMP1 are endoplasmic reticulum (ER)-localizing multi-spanning membrane proteins required for ER-related cellular processes such as autophagosome formation, lipid droplet homeostasis, and lipoprotein secretion in eukaryotes. Both proteins have a VTT domain, which is similar to the DedA domain found in bacterial DedA family proteins. However, the molecular function and structure of the DedA and VTT domains (collectively referred to as DedA domains) and the evolutionary relationships among the DedA domain-containing proteins are largely unknown. Here, we conduct remote homology search and identify a new clade consisting mainly of bacterial PF06695 proteins of unknown function. Phylogenetic analysis reveals that the TMEM41, VMP1, DedA, and PF06695 families form a superfamily with a common origin, which we term the DedA superfamily. Coevolution-based structural prediction suggests that the DedA domain contains two reentrant loops facing each other in the membrane. This topology is biochemically verified by the substituted cysteine accessibility method. The predicted structure is topologically similar to that of the substrate-binding region of Na+-coupled glutamate transporter solute carrier 1. A potential ion-coupled transport function of the DedA superfamily proteins is discussed.