Recent Publications (Original)
Ubiquitination of phosphatidylethanolamine in organellar membranes (Sakamaki et al., Mol. Cell)
2022.09.06 Recent Publications (Original)
Jun-Ichi Sakamaki, Koji L Ode, Yoshitaka Kurikawa, Hiroki R Ueda, Hayashi Yamamoto & Noboru Mizushima
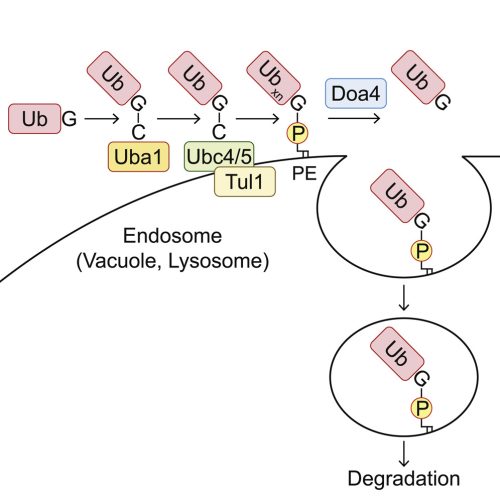
Ubiquitination of phosphatidylethanolamine in organellar membranes
Mol Cell. 2022 Aug 23;S1097-2765(22)00761-4. doi: 10.1016/j.molcel.2022.08.008. Online ahead of print.
The covalent conjugation of ubiquitin family proteins is a widespread post-translational protein modification. In the ubiquitin family, the ATG8 subfamily is exceptional because it is conjugated mainly to phospholipids. However, it remains unknown whether other ubiquitin family proteins are also conjugated to phospholipids. Here, we report that ubiquitin is conjugated to phospholipids, mainly phosphatidylethanolamine (PE), in yeast and mammalian cells. Ubiquitinated PE (Ub-PE) accumulates at endosomes and the vacuole (or lysosomes), and its level increases during starvation. Ub-PE is also found in baculoviruses. In yeast, PE ubiquitination is catalyzed by the canonical ubiquitin system enzymes Uba1 (E1), Ubc4/5 (E2), and Tul1 (E3) and is reversed by Doa4. Liposomes containing Ub-PE recruit the ESCRT components Vps27-Hse1 and Vps23 in vitro. Ubiquitin-like NEDD8 and ISG15 are also conjugated to phospholipids. These findings suggest that the conjugation to membrane phospholipids is not specific to ATG8 but is a general feature of the ubiquitin family.